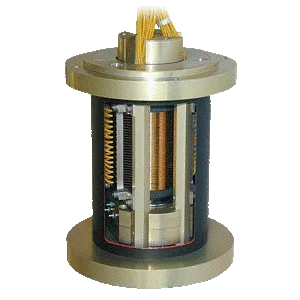
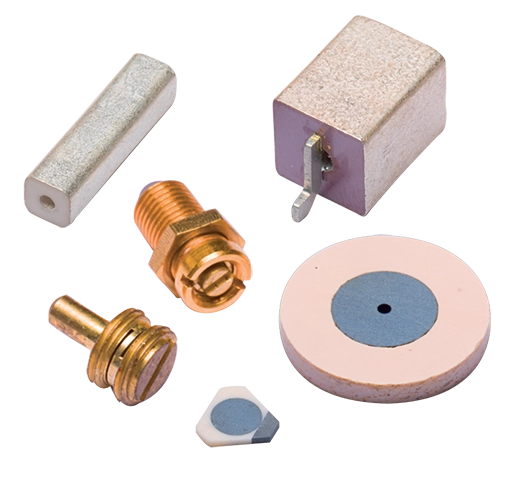
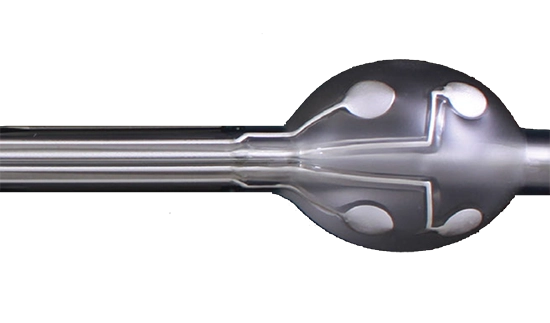
Design it with Micropen
Let the experts at Exxelia Micropen help you with your prototype development, fabrication and concept optimization of components and finished devices for all market segments: Medical Devices, Test & Measurement, and Defense, Military and Space.
Through the ability to dispense many different materials and print on a variety of surfaces, the Micropen technology can be tailored to many different, unique, and challenging applications.

Micropen printing is the most precise and cost-effective way of printing
fine lines of functional materials onto
many different substrates: both 2D
and 3D
surfaces.
We hold 5 certifications :





Exxelia Micropen uses off the shelf materials whenever possible to provide the quickest, least expensive path to a solution.
With over 30 years of experience with the products of all the major ink and substrate manufacturers. For industry-wide applications that do not have a good off the shelf solution we have developed our own in-house material formulation capability.
Inks: Any liquid material is a candidate for Exxelia Micropen printing. Generally, applications call for flowable materials that range in viscosity from 5 to 500,000 centipoise (cP). A wide variety of printing inks have been identified or developed and optimized for both low temperature, flexible, flat and 3D applications, as well as high temperature ceramics, steels and other materials.
- Conductors.
- Insulators.
- Visualization.
- Specialty Inks.

Only Exxelia Micropen has the material science expertise, design engineering expertise and proven success to create the next generation of metallized ceramics.

Exxelia Micropen can accommodate writing on flat substrates
up to 24” x 24” with available systems.
Axial Printing:
Exxelia Micropen routinely processes tubes and rods
from 30 mil (0.030”) diameter up to 6 inch diameter.
Only Exxelia Micropen Technologies has precision printing capability for odd topologies, i.e inside and 3D printing, on any ceramic material, enabling design engineers to push beyond existing boundaries.

Inside Diameter Writing:
Exxelia Micropen has the most advanced precision equipment and experience applying materials on the inside diameter of tubes, ID greater than 0.250.
3D Shapes:
Exxelia Micropen can print on just about any 3D object. If you supply the substrate, we can offer a solution.
Multi-layer Applications:
Exxelia Micropen has experience with integrating multiple materials in a multilayer circuit, interweaving conductive, resistive, and dielectric materials to meet dimensional and performance specifications.


Only Exxelia Micropen Technologies is able to offer end-to-end design engineering product development and fabrication, manufacturing and production for advanced metallized ceramic initiatives.

CoorsTek, CeramTEC, EnRG, etc.
Inks:
Heraeus, Dupont, Ferro, Creative Materials (CMI) etc.
Novel Materials and Applications:
From substrates of standard ceramic and AlN, to inks of Silver, Gold and Platinum, Exxelia Micropen can work with our strategic partners to find your custom solutions.

Our call to “Think it, Design it, Manufacture it” is more than a slogan. It’s how we work with our own teams and partners each and every day to bring your ideas into reality.
At Exxelia Micropen, we integrate material science + design engineering + printed electronics and the result is Exxelia Micropen technology. This proprietary technology is utilized to design and manufacture the most precise and stable designs in the industry.
Micropenning, a “direct write” technology, is Exxelia Micropen’s proprietary technique for manufacturing all of its products. This proprietary technique allows for the deposition of a wide range of material compositions with higher fidelity, accuracy, repeatability, and thickness control than can be achieved by other printing techniques.
At Micropen we have expertise from design through manufacturing and responsive support throughout the product lifecycle. Our flexible printing manufacturing services can meet the needs of any company, from a start-up to a major OEM.
This is especially important in today’s roller-coaster supply chain economy. We have excellent partnerships with suppliers of ink, substrates, and other raw materials and have a well-established process for supply chain management and production scheduling.
The most successful partnerships are long-term and collaborative. The resources on each team should have a shared understanding of the objectives and requirements,
and the chosen supplier should be seen as an extension of your team. This allows challenges to be solved quickly and assures that the project will be completed on time and within budget. Regular meetings, passing files back and forth, and engaging experts in different functions of the organization are all elements of a partnership and lead to the success of the project. Your partner should be proactively communicating throughout the product lifecycle. Your partner may also consider investing in the partnership for the long-term. Over time, as you work together, you’ll be able to apply learnings and efficiency to not only one product but a portfolio of products in a cost-effective and optimized way.
At Exxelia Micropen, we are committed to working collaboratively, starting early in the product design stage. We will share with you our accomplishments, manufacturing controls, and quality assurance process steps. We want you to get to know us, and the earlier we can engage with you as an integrated partner to your design team, the better. Most of our customers have been with us for years and we value a platform approach where we can apply our learnings to product extensions and next-generation designs.
There are complex requirements in the healthcare industry, and a proven track record in the understanding of proper documentation, revision control, and maintenance of device history records and safety regulations will go a long way to ensure that your project is successful. An experienced partner can provide advice on design, suitableinks, techniques, and steps to optimize the process.
This can lead to valuable time and cost savings over the course of the project. There is no substitute for experience in partnering on the design and manufacturing of printed electronics for the implantable medical device market. Exxelia Micropen has over 25 years of experience in working with top-tier medical device companies, printing electronics on endotracheal tubes, ablation devices, balloon catheters, etc.
Our engineering and development teams have the necessary technical expertise, and our production, test, and quality teams understand what it takes to manufacture volume devices while maintaining competitive pricing. We understand that we are adding value to devices that will be used to save or enhance a patient’s life.
50+
Years
Of combined Micropen processing & materials experience
Certified
ISO / EN / AS
All Exxelia sites are ISO9001, EN9100 or AS9100 certified
ISO
13485
certification
Custom
designs experts
from minor catalog product adjustment to 100% custom design based on your specifications
Contact Us
93 Paper Mill Street
Honeoye Falls, NY 14472
Phone: 585-624-2610
Email: micropensales@exxelia.com